Lithium ion batteries have been widely used in portable devices, scientific equipment, space transportation vehicles, and satellite systems due to their high battery voltage and specific energy, wide operating temperature range, long storage life, no environmental pollution, and no memory effect. However, if lithium-ion batteries experience internal short circuits, external short circuits, overcharging, or are used in high-temperature environments, the Joule heat and reaction heat inside the battery will sharply increase, leading to catastrophic hazardous events such as explosions, fires, and thermal runaway.
In order to test the safety performance of lithium-ion batteries, relevant organizations such as Writers Laboratories Japan Battery Association (JBA) and Chinese National Standards (GB) have successively developed lithium-ion battery safety testing standards. The commonly used safety testing items currently include four aspects: thermal performance, mechanical performance, electrical performance, and extreme environmental adaptability testing items.
The nail penetration test is used to evaluate the internal short circuit of lithium-ion batteries caused by lithium deposition, manufacturing defects, or other reasons, or the situation of needle like objects piercing lithium-ion batteries.
1. The significance of nail penetration testing
Nail Penetration Test is an internal short circuit testing method that tests the safety of lithium-ion batteries to withstand internal short circuits. Use steel nails to penetrate the battery, simulate an internal short circuit, and conduct a test to confirm if the battery is smoking, catching fire, or breaking. In addition, nail penetration testing is not only a test to confirm the safety of the battery, but also a test to understand the basic properties of the battery.
Under normal conditions, the positive and negative electrode plates of lithium-ion batteries are insulated by a polymer insulating film in the organic electrolyte – a separator. In this state, inserting the steel nail into the interior of the lithium-ion battery creates a short circuit between the positive and negative electrode plates, forcing an internal short circuit test. The characteristic of this testing method is that it can adjust the testing conditions such as the diameter, material, insertion depth, insertion position, and insertion speed of the steel nails inserted into the battery.
2. The danger of nail penetration
The need for nail penetration testing in lithium-ion batteries means that they are prone to short circuits inside, and if a short circuit occurs, they will be in a very dangerous state. Lithium ion batteries have the characteristics of high energy density, low internal resistance, and allowing for high current flow, making them highly hazardous. Generally speaking, internal short circuits in batteries during use are caused by the presence of conductive foreign objects during the manufacturing process, or by external impact or stress.
In reality, once a product is manufactured, it is difficult to take measures for internal short circuits in control systems, including battery charging and discharging circuits. When an internal short circuit occurs, a huge short-circuit current is generated inside the battery, which in turn generates Joule heat. This heat causes a reaction of flammable organic electrolytes, producing high-temperature gases, and increasing the likelihood of thermal runaway. When thermal runaway occurs, smoke and fire may occur, and in severe cases, it may rupture, endangering the personal safety of the user.
From the user’s perspective, ensuring the safety of the battery is crucial for the application of lithium-ion batteries. Needle testing involves inserting steel nails into the battery, which can easily create an internal short circuit between the positive and negative electrodes. However, when the nail is inserted into the battery to form a hole on the surface of the battery, this hole will release high-temperature gas, causing a change in the internal heat dissipation state of the battery, which may differ from the actual internal short circuit situation.
3. Lithium ion battery nail penetration safety test
The nail penetration test for lithium-ion batteries is conducted using φ 5~ φ 8 mm high-temperature resistant steel needle (with a needle tip angle of 60 ° and a smooth surface without rust, oxide layer, and oil stains) should be inserted at a speed of (25 ± 5) mm/s from the direction perpendicular to the battery electrode plate, and the penetration position should be close to the geometric center of the nail penetration surface (the steel needle stays in the battery). The short circuit inside the battery should be artificially triggered and observed for a period of time. The nail penetration test is shown in Figure 1. If the battery does not catch fire, smoke or explode, it will pass the nail penetration test. Otherwise, it will not pass. The nail penetration experiment mainly studies the effects of nail penetration rate, nail penetration position, state of charge, battery capacity, etc. on battery safety.
During the nail penetration process of lithium-ion batteries, internal short circuits may be caused in four different situations
- - (1) Internal short circuit occurs between the positive and negative current collectors (aluminum foil and copper foil)
- - (2) Occurred between aluminum foil and negative electrode
- - (3) Occurred between the positive and negative electrodes
- - (4) It occurs between the copper foil and the positive electrode gate.
On the other hand, during the nail penetration process of lithium-ion batteries, more than one type of internal short circuit is often triggered, and the situation of internal short circuit will also evolve over time. This is the underlying reason for the unclear mechanism of internal short circuit and poor repeatability during the nail penetration testing process of lithium-ion batteries.
According to current understanding, the basic process of internal short circuit caused by lithium-ion batteries during the nail penetration process is as follows: Firstly, the Joule heat generated by the internal short circuit causes a rapid increase in the local temperature of the battery. After the temperature reaches a certain value, it causes the decomposition of the SEI membrane (80-120 ℃) and the melting of the membrane (165 ℃). The decomposition of SEI membranes and the melting of membranes generate more heat, which promotes electrolyte decomposition (130-300 ℃) and negative electrode reduction reaction (100-400 ℃), while positive electrode oxidation reaction (160-400 ℃) ultimately leads to uncontrolled heating.
The parameters that need to be tested in the safety testing of nail penetration include
- - (1) Temperature changes at different positions of lithium-ion batteries during nail penetration process
- - (2) Voltage changes in lithium-ion batteries during nail penetration process
- - (3) The self heating rate, initial thermal runaway temperature, reaction level, and Arrhenius coefficient of lithium-ion batteries during the piercing process.
These parameters are used to analyze the possible reactions that may occur during the nail penetration process of lithium-ion batteries, as well as the occurrence of thermal runaway.
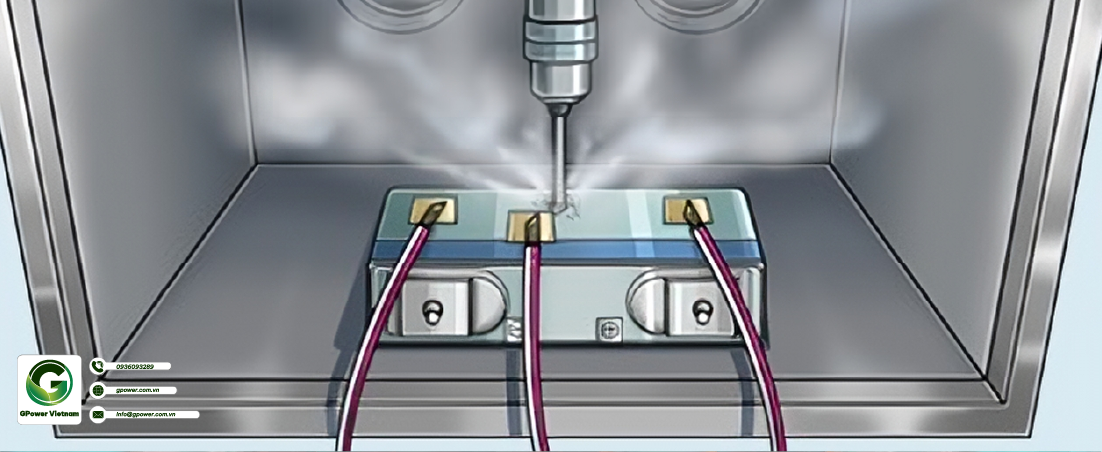
4. Actual test results
Conducted a nail penetration test on a 18650 lithium-ion battery with a capacity of 22 Ah and found that as the nail penetration rate increased, the probability of the lithium-ion battery passing the safety test increased.
After studying the effect of needle speed on the safety of lithium-ion batteries, it is believed that needle speed has a relatively small impact on the needle safety of cylindrical batteries; And it has a significant impact on the safety of soft pack power batteries. Specifically, the higher the needle penetration speed, the greater the possibility of thermal runaway of the battery. Some people believe that when the needle penetration speed is slower, the local heat generation of the battery is higher.
It can be seen from this that the conclusions of the above three works are not consistent. There are many reasons that can cause this situation.
- - Firstly, the winding and stacking structures are different, with the winding type battery having tighter contact between the layers.
- - Secondly, when the nail penetration speed is low, on the one hand, the extensibility of the diaphragm protects the battery and prevents the occurrence of internal short circuits. On the one hand, after an internal short circuit occurs, the duration of local high current increases.
- - In addition, different thicknesses of copper foil, aluminum foil, positive and negative electrodes, and separators can also lead to different test results at different needle piercing speeds.
Conducted needle piercing tests on fully charged lithium-ion batteries using cubic steel needles with dimensions of 40 mm x 1.5 mm x 1.5 mm from different positions of the battery. They found that the position in the middle of the green edge of the battery, far away from the pole ear direction, caused the greatest temperature rise and had the worst safety. They believe that the main reason for this phenomenon is the poor thermal conductivity of the battery edge separator, which limits the thermal dissipation of lithium-ion batteries.
Conducted nail penetration tests on 18650 lithium-ion batteries with a nominal capacity of 22 Ah under different states of charge (SOC). It was found that as SOC decreases, the probability of lithium-ion batteries passing safety tests increases. This is because the higher the state of charge, the higher the initial voltage of the battery. This further increases the internal short-circuit current and prolongs the short-circuit time. As a result, the safety of lithium-ion battery nail penetration testing becomes worse.
Conducted a nail penetration analysis on fully charged 604-1104 m Ah lithium-ion batteries and found that the higher the battery capacity, the worse the safety of nail penetration testing for lithium-ion batteries.
In addition, conducted nail penetration testing analysis on polymer lithium-ion batteries using ceramic separators. They collected the temperature of multiple batteries with different SOC in the nail penetration area and battery surface, the voltage changes of the batteries, and the burr state of lithium-ion batteries after nail penetration. They analyzed the mechanism of nail penetration based on this. It is believed that the process of needle piercing through the battery causes aluminum burrs and copper burrs to connect, forming an internal short circuit between aluminum foil and copper foil.
The temperature of the local short-circuit zone increases with the generation of Joule heat. If the temperature reaches the melting temperature of aluminum, the aluminum burrs will melt and burn, causing a circuit breaker with the copper burrs. They can be summarized into three models:
- - Model A, where the aluminum burrs melt and the copper burrs no longer come into contact.
- - The aluminum burrs in Model B did not melt and formed an internal short circuit in contact with the copper burrs.
- - The aluminum burrs in Model C do not completely melt, and after a period of time, they come into contact with the copper burrs again, forming an internal short circuit.
They believe that changing the combustion and melting of aluminum burrs is a new direction for battery safety design.
Not only recorded the temperature near the battery needle, electrode temperature, and battery voltage in the nail penetration experiment, but also recorded the pressure changes on the battery surface and found a clear correspondence between the pressure peak on the battery surface and the temperature peak on the battery.
They believe that in addition to battery voltage and temperature, battery safety can also be described by increasing other measurement quantities such as pressure. In the above experiment, people can analyze the nail penetration of lithium-ion batteries more realistically through experimental methods. However, the type of short circuit that occurs inside the battery during the nail penetration test is only speculation and does not have good theoretical support. The nail penetration model of lithium-ion batteries is another method to analyze the nail penetration mechanism of lithium-ion batteries and improve their safety performance.
5. Blunt nail test
The mandatory internal short circuit test uses a spherical pin at the top, which is a testing method that can create an internal short circuit (small short circuit) without causing perforation on the battery surface, known as the Blunt Nail Test. This method creates a short circuit between the electrode materials (positive and negative electrode plates) of the battery through the pressure of nails, resulting in only a slight deformation of the tested battery. Compared to the usual nail penetration test, this method can create a state closer to the actual internal short circuit.
6. Preparation of test chamber
To conduct this test, manually inserting nails into lithium-ion batteries is definitely not feasible. Do not attempt it easily – nails may fly out, causing hand and body injuries. Therefore, it is necessary for us to use a needle testing machine. The DGBELL nail penetration testing chamber used here (with a maximum pressure of 20 k N and a stroke of 200 mm) has been made to be able to install steel nails of two different diameters( φ 3 mm φ 5 mm, 100 mm).
7. Security measures
For safety reasons, the testing machine has an explosion-proof function to prevent battery fires and explosions from endangering the safety of testing personnel. Due to price considerations, the testing machine has options for hydraulic motors and computer servo motors. The hydraulic motors are easy to operate, while the servo motors can be set with more detailed testing conditions, resulting in higher accuracy.
Equipped with a temperature and voltage acquisition system, the battery temperature data is collected through K-type thermocouples, plug and play, with a measurement range of 0-600℃. The voltage collection range is 0-50 V. The test box is equipped with fixtures that can fix batteries and steel needles of different sizes. The insertion speed of the nail is adjustable from 0.1 to 80 mm/s.
8. Related precautions
Here, we test three different types of stacked batteries (300 m Ah, 1000 m Ah, 2000 m Ah). The data to be measured is as follows:
- - The variation of battery terminal voltage over time
- - Battery surface temperature
- - Changes in the appearance and shape of the battery after nail penetration
Before conducting the experiment, it is necessary to carefully prepare fire prevention and safety measures, such as preventing splashing, leaving enough maintenance space for the testing machine, and ensuring that the fire extinguishing system can operate normally.
9. Differences caused by nail penetration conditions
Based on several experimental results, including the above test results, the following facts can be inferred.
- - (1) At the puncture point, the terminal voltage drops significantly and gradually drops to 0 V. When the voltage drops to a certain extent, there will be significant fluctuations in the voltage curve.
- - (2) At the stage of significant voltage changes from the needle point to the terminal, the test results of different battery types and needle conditions also vary, but the terminal voltage will drop to 0 V after 10-20 seconds.
- - (3) When testing batteries of the same capacity, the thicker the nail, the greater the change in terminal voltage.
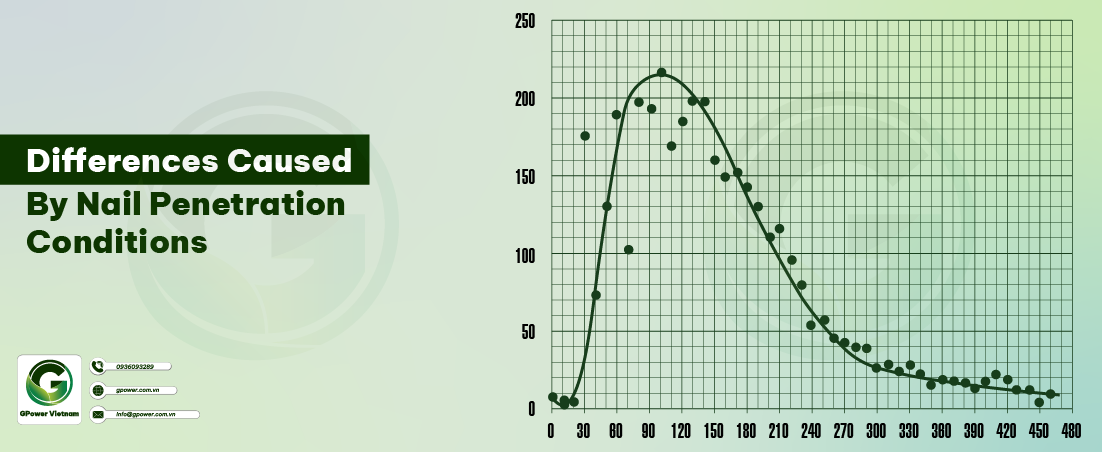
10. Measurement of battery surface temperature
After nail penetration, the surface temperature of the stacked battery can be obtained over time through a temperature acquisition system. Using φ The surface temperature change measured during the puncture test of a 2000 m Ah stacked battery with a 5 mm steel nail.
11. Temperature rise after nail penetration
During the puncture test of a 2000 mAh stacked battery with a 5 mm steel nail, there was a situation of accompanying fire and temperature exceeding 200 ℃. In addition, in the testing of 1000 mAh battery samples φ A fire broke out when a 3 mm steel nail was punctured. Using φ In the puncture test conducted with a 5 mm steel nail, not only did a fire occur, but the battery temperature also increased significantly.
Test multiple battery samples using steel nails with different diameters, and the trend of temperature rise over time is similar to the above figure. Moreover, the highest temperature measured for different battery cells can also be below 100℃.
This test, by short circuiting the positive and negative electrodes, instantly converts the stored electrical energy inside the battery into thermal energy, generating enough heat to cause the electrolyte to burn and explode. From an energy perspective, the heat generated by igniting gasoline is almost the same.
Lithium-ion batteries are safe to use within the manufacturer’s specified rated range. Exceeding the rated range, such as repeated overcharging or discharging, can cause the battery to catch fire or be damaged.





